Research
Hypothalamic behavioral state control
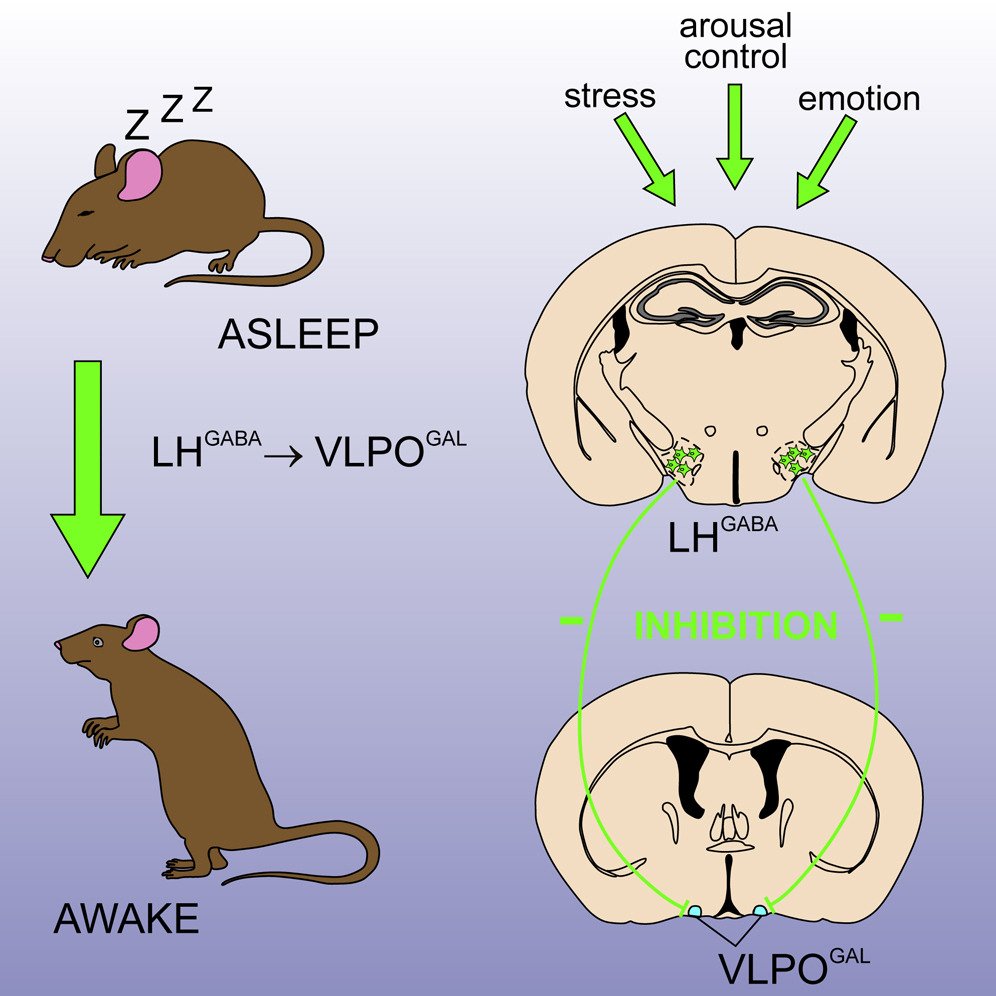
The lateral hypothalamus (LH) has historically been recognized as a crucial area for promoting arousal, orchestrating behaviors necessitating active wakefulness via excitatory projections to diverse arousal and reward centers in the brain. Recent research, including discoveries from our laboratory and collaborations with the Arrigoni laboratory, has revealed that inhibitory neurons originating from the LH and their projections also suppress a key preoptic sleep-promoting center, thereby inducing arousal. Building upon these insights, our work aims to advance future therapeutic and interventional targets for treating neuropsychiatric, neurodegenerative, and sleep-wake disorders, including insomnia.
Regulation of arousal and sleep by the Suprachiasmatic Circadian Clock
Have you ever wondered why you're able to take a nap in the afternoon but not in the evening? Or why it's difficult to fall asleep early when traveling to a time zone further east? It has long been known that the circadian clock strongly promotes arousal towards the end of the wake period. However, almost nothing is really known about which neurons in the brain's circadian clock, the suprachiasmatic nucleus, control this phenomenon and what brain circuitry is involved. This project aims to address this gap.
Brainstem Slow-Wave Sleep Promoting Parafacial Zone
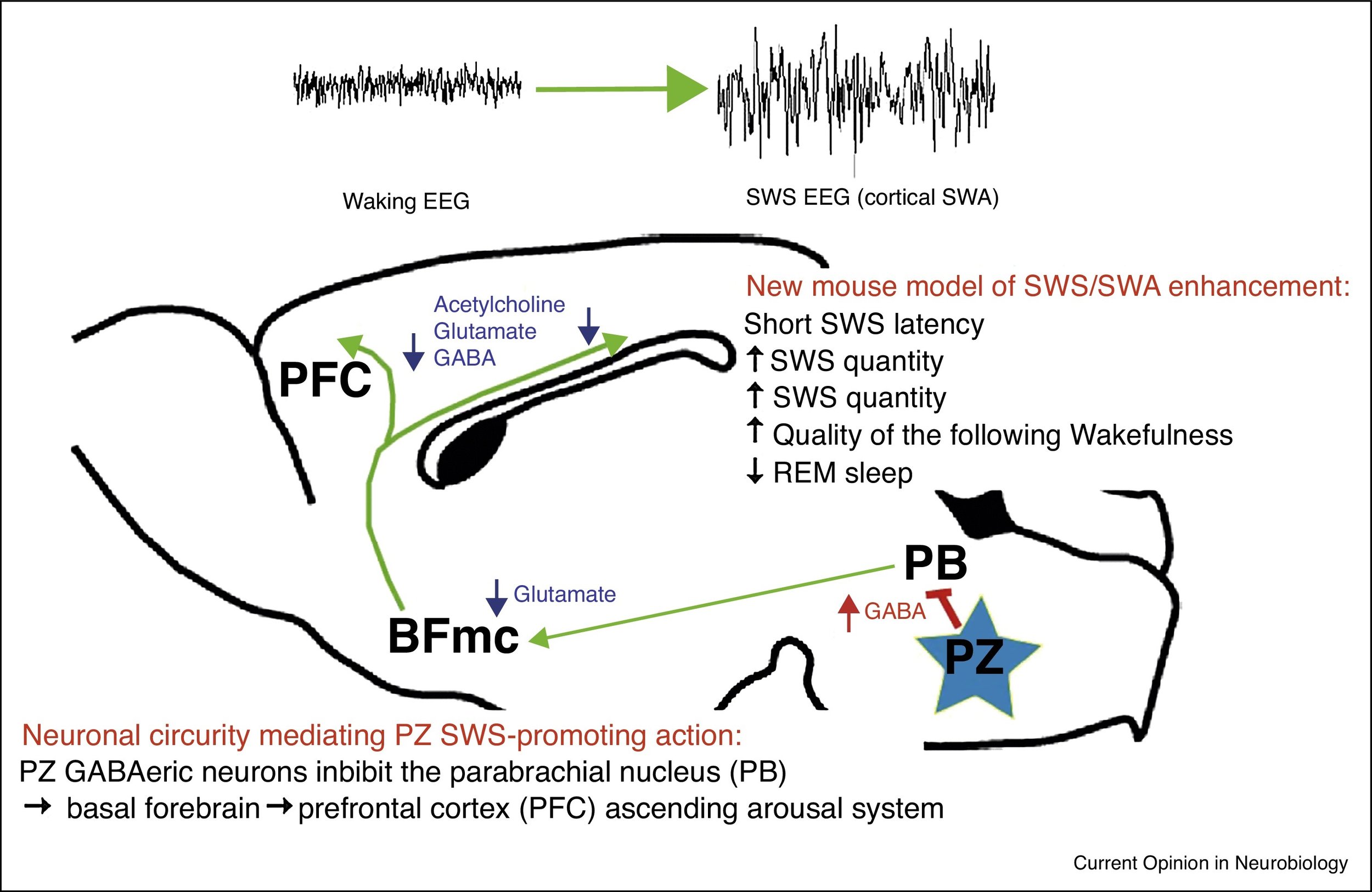
Discoveries made in the early 20th century suggested the existence of a sleep-promoting area in the brainstem. Legacy techniques, such as targeted induced neuronal lesions, couldn't elucidate this area due to the complex, reticulated structure of the vital functions controlled by the brainstem. However, with the advent of cell-type-specific targeting in the late 2000s, this began to change. We discovered a group of sleep-active and slow-wave sleep-promoting neurons lateral to the facial nerve (VII), which we coined the 'parafacial zone.' Please note that this 'parafacial zone' should not be confused with the one that regulates breathing and is located medial and caudal to the sleep-promoting 'PZ.' Ongoing work involves the molecular characterization of these neurons and what their projections throughout the neuraxis can teach us about the control of NREM sleep.
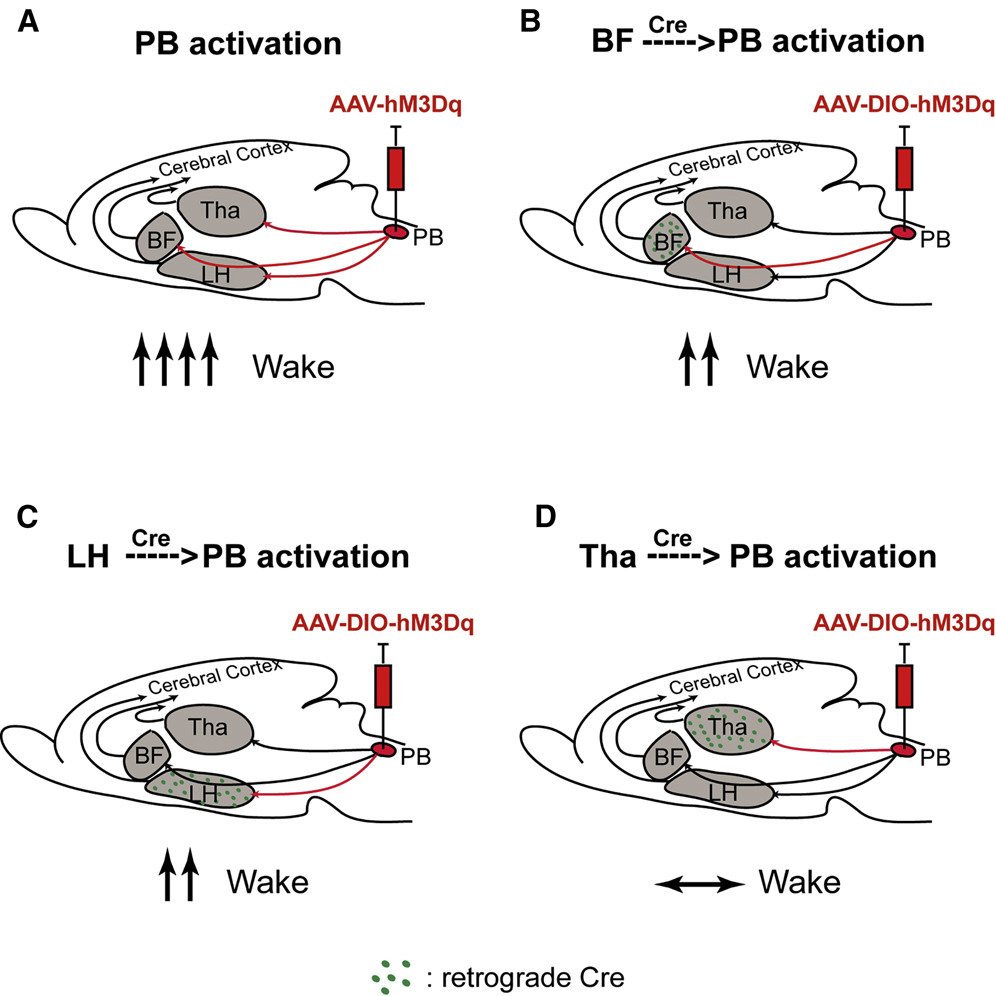
Parabrachial FoxP2
Obstructive sleep apnea (OSA) is a common disorder marked by airway obstructions during sleep, linked to various health issues. Restoring airway patency often disrupts sleep, complicating treatment. Efforts to pharmacologically enhance ventilatory drive have been limited by increased arousal. This study aims to identify CO2-responsive FoxP2 neurons in the brainstem's lateral crescent parabrachial nucleus (PBclFoxP2) as potential targets to enhance ventilation without causing arousal. The research will map forebrain inputs to PBclFoxP2 neurons, uncover unique receptors, study presynaptic activity, and assess the impact on ventilatory response to hypercapnia. By delineating these circuits, this study aims to contribute to developing pharmacological interventions for OSA, advancing patient care and treatment outcomes.
Sleep circuitry and cognitive function in Alzheimer’s Disease
This project investigates the molecular, cellular, physiological, and behavioral effects of acute or chronic sleep extension by harnessing the potent slow-wave sleep-enhancing effect achieved through chemogenetic activation of the brainstem parafacial zone (PZ)
Adaptation to Artificial Gravity in Low Earth Orbit
One of the numerous challenges inherent in long-duration spaceflight, such as missions to Mars, the Moon, or prolonged stays at the International Space Station (ISS), is overcoming the physiological stress of chronic lack of gravity on the body. Many of these challenges faced by bipedal terrestrial mammals, such as humans, can be modeled in quadrupedal laboratory rodents, like the fluid shift to the head. In this project, we assess the effectiveness of providing 'artificial gravity' to mice housed on the ISS to counter the effects of prolonged microgravity.
Techniques
Techniques
The Fuller laboratory is a leader in technological innovation in systems neuroscience. We collaborate with experts from around the world and are committed to driving innovation in the rapidly growing field of biomedical technologies in the neurosciences.
In our work, we primarily use genetically-driven targeting and engage in the development of cutting-edge methodologies. Our techniques cover a wide range, including:
Morphological methods
Genetic engineering techniques in mice and rats
Brain slice electrophysiology
In vivo single-cell and population Ca2+-based imaging
Optetrode/LFP recordings
Optogenetics
Chemogenetics
Single-neuron and single-nuclei transcriptomics
Novel intersectional vector approaches
Cell-specific mapping techniques
Additionally, we operate as a shareware lab, so don't hesitate to ask for a reagent – no strings attached! Contact us.